
February 16, 2026
News
Potential big news for the 40 million Americans with migraine.
February 16, 2026
It is long overue to make triptans available without a prescription, like in the rest of the world.
Read article

January 12, 2026
Research
Rethinking Migraine Risks: Are Triptans Safer Than We Thought and CGRP Drugs Less So?
January 12, 2026
Triptans are safer than thought, while CGRP drugs are less so.
Read article
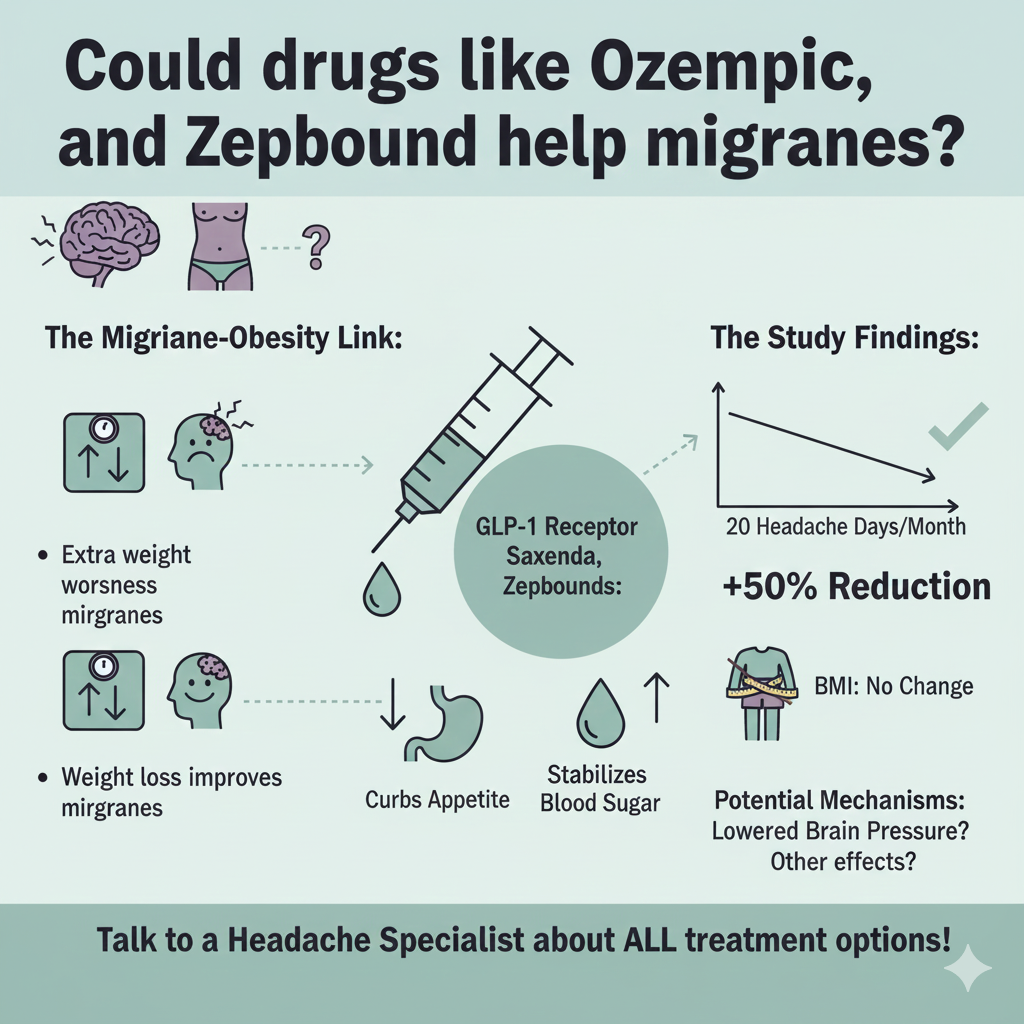
December 15, 2025
Alternative Therapies
Could drugs like Ozempic, Saxenda, and Zepbound help migraines?
December 15, 2025
Could drugs like Ozempic, Saxenda, and Zepbound help migraines? Extra weight often increases migraine headache frequency and makes standard treatments less effective. But a study just published in the journal Headache shows that a medication commonly used for weight loss and diabetes, liraglutide (Saxenda), might offer relief for tough-to-treat migraines in people with obesity.
Read article

November 15, 2025
Cluster headaches
Cluster headaches and solar activity
November 15, 2025
It was an unusual week at the New York Headache Center. After months of relative calm, my schedule suddenly filled with cluster headache patients—one even consulting me virtually from Saudi Arabia. The influx came right after a G5-level geomagnetic storm, one of the strongest solar events in recent memory.
Read article

November 10, 2025
Alternative Therapies
A Week of Meditation Changes Brains and Bodies
November 10, 2025
A week-long meditation retreat produces dramatic changes in brain and metabolic functions
Read article

October 21, 2025
Alternative Therapies
Meditation is better than slow breathing exercise in reducing pain
October 21, 2025
A new study published in the journal PAIN by Dr. A. Amorim and her colleagues at the University of California San Diego examined how mindfulness meditation reduces pain. The findings help clarify whether mindfulness meditation is more effective than simple slow breathing for pain relief.
Read article

September 7, 2025
How Artificial Sweeteners May Be Affecting Your Brain
September 7, 2025
A large Brazilian study published in Neurology followed nearly 13,000 adults for eight years and found something troubling: people who consumed the most artificial sweeteners showed faster cognitive...
Read article

August 30, 2025
Lithium Levels are low in Alzheimer’s. Is there a role in Migraine and Chronic Pain?
August 30, 2025
Recently published research on lithium deficiency in Alzheimer’s disease has caught the attention of the media. As a neurologist specializing in pain and headache medicine with an interest in no...
Read article

August 16, 2025
The Healing Power of Holding Hands: Insights from Neuroscience on Pain Relief
August 16, 2025
There’s something powerful about human touch when you’re hurting. As a neurologist, I see every day how a gentle hand squeeze from someone you trust can shift your pain—not just emotionally, but in...
Read article

August 7, 2025
Virtual Reality Nature Experiences May Help Reduce Pain Sensitivity
August 7, 2025
In my latest book, The End of Migraines: 150 Ways to Stop Your Pain, I mentioned studies that showed the pain-relieving effect of virtual reality. New research reveals how immersive natural environments...
Read article

July 21, 2025
Research
Magnetic Brain Stimulation Helps Stroke Survivors Improve Speech
July 21, 2025
Aphasia, a language disorder that often follows stroke, affects millions of people worldwide. It can make speaking, understanding, reading, and writing difficult or even impossible. For many, recovery stalls after the first few months, leaving…
Read article

July 10, 2025
Update
Triptans in Pregnancy: New Study Finds No Long-Term Developmental Risks
July 10, 2025
Many women with migraine worry about taking their medications during pregnancy. Migraine drugs in the triptan family (like sumatriptan, rizatriptan, etc.) are very effective for stopping attacks, but questions have remained about their safety for…
Read article

July 7, 2025
Insight
TMS for Mild Cognitive Impairment
July 7, 2025
Mild cognitive impairment (MCI) is a term used by neurologists to describe symptoms such as forgetting names, losing focus, or struggling with simple tasks. While MCI involves subtle changes in memory or thinking that don’t…
Read article

June 23, 2025
USC research suggests that magnesium can help post-concussion syndrome
June 23, 2025
Researchers at USC’s Keck Medicine presented a poster with their findings at the Annual Scientific Meeting of the American Headache Society in Minneapolis, which concluded yesterday. They described the effect...
Read article

June 15, 2025
New Migraine Medications Still Underused. Work best when combined with Botox
June 15, 2025
A large new study led by Dr. Sait Ashina reveals that revolutionary migraine prevention medications remain significantly underutilized, even though they represent a major breakthrough in migraine...
Read article

June 9, 2025
Vitamin D: The Overlooked Key to Brain Health – From Migraines to Multiple Sclerosis to Immune Defense
June 9, 2025
For over 15 years, research has been building a compelling case that vitamin D plays a crucial role in neurological health, far beyond its well-known effects on bone health. Recent groundbreaking studies are...
Read article

June 5, 2025
Do Migraines Really Damage the Brain? New Research Challenges Old Beliefs
June 5, 2025
For years, doctors and patients have worried that migraines—especially those with aura—might leave behind silent “scars” in the brain, visible as white spots (called white matter hyperintensities, or WMHs) on MRI...
Read article

May 30, 2025
Concussion Headaches are Like Migraines
May 30, 2025
Scientists at Copenhagen University Hospital compared 132 adults who still had headaches a year or more after a mild traumatic brain injury (called persistent post-traumatic headache, or PTH) with 751 people...
Read article

May 21, 2025
Transcranial Magnetic Stimulation for Pain
May 21, 2025
We have been using repetitive transcranial magnetic stimulation (rTMS) to treat chronic migraine headaches and other types of pain and neurological disorders. An article published in Pain Medicine News provides...
Read article

May 8, 2025
Breakthrough in Treating Posttraumatic Headache with TMS
May 8, 2025
At the New York Headache Center, we have been using fMRI-guided repetitive Transcranial Magnetic Stimulation (rTMS) to treat chronic migraine, posttraumatic headaches (PTH), pain syndromes, and neurological...
Read article

May 3, 2025
New Study: Over-the-Counter Pain Relievers Speed Up Concussion Recovery
May 3, 2025
A major new study offers hope for athletes and others recovering from concussion: using over-the-counter (OTC) analgesics like acetaminophen and NSAIDs may significantly improve symptoms and reduce...
Read article

April 20, 2025
Flossing teeth: a surprising link to fewer strokes and migraines
April 20, 2025
You may think of flossing as a way to keep your teeth and gums healthy, but new research shows that it may also protect your heart and brain. Recent scientific studies reveal compelling connections between gum health...
Read article

April 13, 2025
fMRI changes in kids after a concussion; could TMS help?
April 13, 2025
Concussions in children are far more than just a bump on the head—they can trigger subtle yet significant changes in brain function that may linger long after the visible symptoms fade. A recent prospective, longitudinal...
Read article

April 5, 2025
Zonisamide Shows Promise as a Preventive Migraine Treatment for Kids and Teens
April 5, 2025
A new study suggests that zonisamide (Zonegran), a medication traditionally used to treat seizures, may help reduce migraine days in children and teens. The research, led by Dr. Anisa Kelley of Northwestern University Feinberg...
Read article

March 28, 2025
My book, The End of Migraines: 150 Ways to Stop Your Pain, just became available as an audiobook on Amazon.
March 28, 2025
Check it out https://a.co/d/gwB2xEw...
Read article

March 23, 2025
Medical Cannabis vs. Prescription Medications for Chronic Pain
March 23, 2025
In a recent study published in Pain by the University of Pittsburg researchers, the effectiveness of medical cannabis was compared with traditional prescription medications for managing chronic pain...
Read article

March 17, 2025
The Right Tempo in Music May Help Reduce Pain
March 17, 2025
New research from McGill University published in the journal Pain suggests that the tempo—or speed—of music plays a crucial role in how well it helps reduce pain. In this study, researchers explored whether music that matches...
Read article

March 9, 2025
Meeting of the Headache Cooperative of the Northeast
March 9, 2025
It was an honor to participate in the 35th Annual Winter Symposium of the Headache Cooperative of the Northeast, held on March 7 and 8. Leading headache experts from across the country covered many interesting topics...
Read article

February 27, 2025
Vitamin B12: “Normal” blood Levels are often not normal
February 27, 2025
A new study published in the Annals of Neurology by researchers at UCSF challenges our current understanding of what constitutes “normal” vitamin B12 levels, particularly regarding brain health. The findings suggest that...
Read article
.png)
